Treatment Option for Hep C in Pediatric Patients - FDA Approval
The safety and efficacy of Harvoni have also been studied for the treatment of HCV genotypes 4, 5 or 6 infection in pediatric patients of 12 years of age and older and the obtained data showed the similar efficacy as in adult patients. In addition, Harvoni quantity in the body of the pediatric patient for treating of HCV genotypes 4, 5 or 6 infection is same as in HCV genotype 1 infection. This means Harvoni provides similar efficacy in every targeted HCV genotypes. Overall the common adverse reactions of Harvoni reported in the clinical trial were fatigue and headache. [1]
Detail Report of Clinical Trial Conducted on Sovaldi with Ribavrin
• Type of Trial: Open-label, multicentric clinical trial [1]
• Number of patients Enrolled: 50 [1]
• Inclusion criteria: pediatric patients with 12 years of age and older or weighing at least 77 pounds (35 kilograms). The calculated average age of the children is 15 years old. [5]
• Gender specification upon the selected patients: 42% of the subjects were female [5]
• Selected Drugs: Sovaldi in combination with ribavirin [1]
• Treatment regimen: Sovaldi and Ribavirin are oral pills (the daily dosage of ribavirin is weight-based and is administered orally in two divided doses with food.) [1]
• Treatment duration: 12 weeks for genotype 2 with/without cirrhosis or with compensated (mild) cirrhosis of 12 weeks. [1]
However, for genotype 3 without cirrhosis or with compensated (mild) cirrhosis was for 24 weeks.[7]
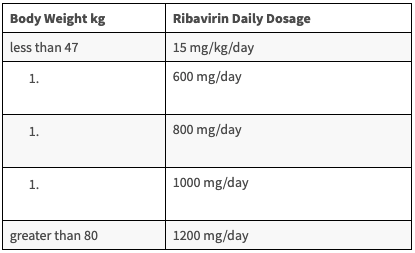
• Dose: The recommended dosage of SOVALDI in pediatric patients of 12 years age and older or weighing at least 35 kg is one 400 mg tablet taken orally once daily. The daily dosage of ribavirin is weight-based and is administered orally in two divided doses with food. [5]
Table: Recommended Dosing for Ribavirin in Combination Therapy with SOVALDI for Pediatric Patients 12 Years of Age and older or Weighing at least 35 kg [5]pliance-remains-high
FDA reported that the efficacy results evaluated through an open-label clinical trial were comparable to those in adults. Furthermore, the efficacy results also showed that 100 percent pediatric patients (aged 12 years and older) with Hep C genotype 2 and 97 percent of pediatric patients (aged 12 years and older) with Hep C genotype 3 had no detectable virus identified in their blood sample after completing treatment duration. [1]
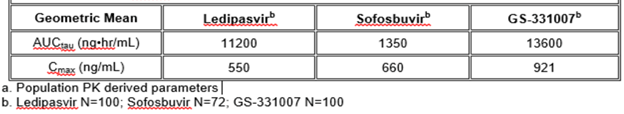
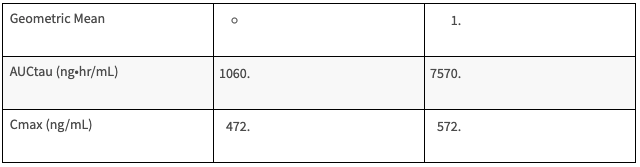
Table: Pharmacokinetic Properties of SOVALDI in HCV-infected Pediatric Subjects 12 Years of Age and Older [5]
Fatigue and headache were the reported adverse events in Hep C treatment with Sovaldi in combination with ribavirin. In addition, all ribavirin-related contraindications are even applicable for Sovaldi combination therapy. [1]
The risk associated with this treatment option is the reactivation of Hepatitis B virus (HBV) during or after completing the treatment regimen of direct acting anti-viral drugs, as also reported in the case of adult patient's treatment, specifically for without administering HBV antiviral therapy. The outcome of the reactivation of Hepatitis B virus (HBV) leads to serious liver problems or death. Therefore, it has been suggested by FDA that the Healthcare professionals should screen all patients for evidence of current or prior HBV infection before starting treatment with Harvoni or Sovaldi. [1]
This much awaited and appreciable approval can obviously be able to change the HCV treatment scenario by attaining an unmet requirement of children and adolescent. In addition, FDA approval can render to get easy insurance coverage.
References
1. FDA approves two hepatitis C drugs for pediatric patients. FDA News Release. U.S. Food and Drug Administration. Content last updated on April 7, 2017. Online available at https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm551407.htm
2. Michael R. Narkewicz. American Liver Foundation. Content last updated on July 19, 2016. Online available at http://hepc.liverfoundation.org/what-is-hepatitis-c/hepatitis-c-in-children/
3. Hepatitis C Virus Infection Among Adolescents and Young Adults --- Massachusetts, 2002--2009. Centers for Disease Control and Prevention. Content last updated on May 06, 2011. Online available at https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6017a2.htm
4. Vinod K Dhawan. Hepatitis C Treatment & Management. Medscape. Content last updated on Mar 28, 2016. Online available at http://emedicine.medscape.com/article/177792-treatment
5. FDA Approves HCV Sovaldi and Harvoni For Children Ages 12 to 17. HCV New Drugs. Content last updated on April 7, 2017. Online available at http://hepatitiscnewdrugs.blogspot.in/2017/04/fda-approves-hcv-sovaldi-and-harvoni.html
6. Mitchel L. Zoler. VIDEO: Harvoni shows safety, efficacy in adolescents for hepatitis C. Pediatric News. Content Published on October 11, 2016. Online available at http://www.mdedge.com/pediatricnews/article/115427/adolescent-medicine/video-harvoni-shows-safety-efficacy-adolescents
7. FDA Approves Hepatitis C Treatment Breakthrough for Children. Daily Life. Online available at https://www.lifebeyondhepatitisc.com/2017/04/fda-approves-hepatitis-c-treatment-breakthrough-children/
Blog Categories
Stay Informed