The Essential Role of Cleaning and Sanitization in Pharmaceutical Manufacturing
The cleaning validation guidelines by the FDA require that pharmaceutical manufacturers not only sanitize equipment to prevent contamination but also thoroughly establish written procedures for doing so. Good manufacturing practices apply especially to the medical industry to ensure safety and effectiveness in the drugs, medical devices, and other products put on the market.
Because of the high penalties for noncompliance and the great reputational risk, all pharmaceutical manufacturers must make serious efforts to stick to cleaning validation guidelines.
While there have been multiple peer-reviewed studies on the topic, this article will cover the basics of cleaning, sanitization, and validation in the context of medical manufacturing.
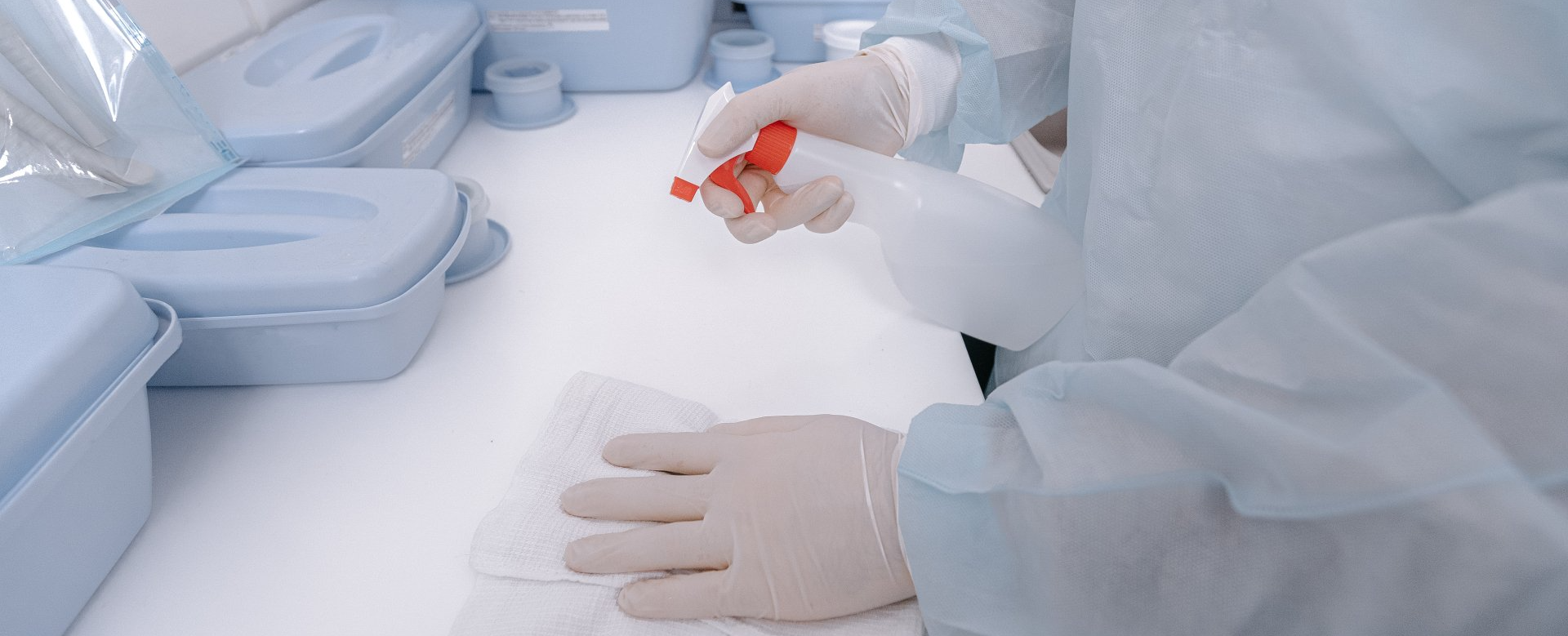
Why Pharmaceutical Facility Sanitization Matters
It’s no secret that a clean workstation matters, as any office worker or kitchen chef can attest to. The maintenance of equipment and workstations is especially important in the medical field for obvious reasons. Preparing the workstation of a medical manufacturing facility involves the following.
- Cleaning: In the context of medical manufacturing, cleaning refers to the removal of dirt, debris, and residues from every piece of medical equipment, supplies, and environment. The effectiveness of cleaning procedures is determined by a visual inspection and then verified through laboratory testing.
- Sanitation: As a related concept, sanitation has to do with clearing out microbiological contamination, usually through a chemical sanitation process. Most regular cleaning procedures are not intended to cover microbial contaminants. And, since pharmaceutical manufacturing facilities need a relatively clean equipment surface before you start sanitizing, regular cleaning is usually done first.
- Sterilization: The environment and air quality of the facility matters too, hence why these companies invest in High-Efficiency Particulate Air (HEPA) filters and give some rooms positive air pressure to prevent outside contaminants from entering through the air.
Of course, the entire process is far more complicated than what is described here. For instance, your choice of cleaning agent matters, depending on the nature of the surface and the contaminants. What temperature is optimum for its use? How long is the exposure period? What should the pH and concentration be?
It’s all worth it in the end to avoid biological, chemical, or environmental contamination from messing with the safety and effectiveness of the final product.
What Procedures Must Be Set in Stone?
One of the requirements of FDA cleaning validation is clearly establishing and writing down proper maintenance procedures that staff on the factory floor must adhere to. Some examples in this regard are:
- Assignment of responsibility for the inspection of equipment to clean each piece of equipment and determine cleanliness
- Cleaning schedules for maintaining and sanitizing the workplace
- Detailed procedures of disassembling and setting back up certain equipment for deep cleaning
- Detailed sanitization procedures, tools, and sanitizing agents to be used
- Pharmaceutical decontamination protocols and preventative measures to protect equipment from contamination
- Formal inspections of factory equipment
- Records of maintenance, inspections, and cleaning
Ensuring a safe environment for drug and device production relies on proper procedures for inspecting and documenting cleaning activities.
What Does “Cleaning Validation” Entail According to the FDA?
Validation is the act of showing that cleaning procedures are effective for a particular use case within a particular environment. You must specify these details in your written procedures and ensure that they are followed precisely in the field.
- Strength of the sanitizing agent
- Concentration of the agent and the solvent used
- Temperature
- pH
- Procedure for disassembling equipment for cleaning
For instance, while it may make sense to use more of a cleaning agent, doing so may cause an imbalance in the pH or mess with the exact concentration necessary for the job. The result is an invalid cleaning procedure.
What Types of Contamination Should Medical Production Facilities Look For?
Cleaning validation for medical devices, drug product containers, and packaging materials primarily aim to prevent contamination in the first place rather than respond to it afterward. There are many ways for contaminants to introduce themselves into your production line.
- Biological: Much like with food products, micro-organisms like bacteria, molds, and viruses may end up in the final product and render it unsafe for use. Your choice of sanitizing agent matters just as much as your cleaning procedure to prevent biological contaminants.
- Chemical: You introduce the risk of chemical contamination whenever different products are processed using the same equipment without proper sanitization beforehand. There’s even a risk when your cleaning agents come into contact with the product as well. The best defense against this problem is to validate the cleaning procedure by following the written instructions exactly.
- Environmental: Some steps in the manufacturing process, like bottle filling and packaging, put the final product in contact with the external environment. Sterilization of the air in the facility matters for this reason.
Sanitization works as a pre-emptive measure against contamination because prevention is the best defense in medical manufacturing. Visual inspections cannot reliably detect contamination in pharmaceuticals, as most contaminants are invisible to the eye and difficult to detect.
Also, steroids, antibiotics, hormones, and other similar materials can all cause significant changes in the body even in small amounts. For this reason, facilities working with them need to be extra careful to avoid cross-contamination by sanitizing equipment regularly and isolating such materials during the production line.
How Should a Sanitizing Agent Be Chosen?
There are many different types of agents to use for pharmaceutical facility sanitization, and the choice of which one to use comes down to particular circumstances. However, you almost never stop at using just one.
Sanitizers kill the majority of bacteria on most surfaces. They are applied after initial cleaning and, according to good manufacturing practices, can include 70% alcohol, chlorines, QUATs, hydrogen peroxide, and sodium hydroxide.
Other options exist as well for different purposes. Disinfectants kill certain bacteria, viruses, and fungi. And sporicide kills bacterial spores.
What Role Does Personal Hygiene Play in a Facility?
While sanitizing workstation equipment and the facility environment matters, don’t forget to educate staff members on their own cleanliness. For example:
- Wearing hygienic clothing like gloves and protective equipment when working with, storing, or loading medical materials.
- Segregating clean and dirty clothing and working with a certified contractor or in-house facility for laundering the clothes.
- The use of hand sanitizers and disinfectants
Getting staff members on board with your sanitization efforts is one of the best ways to ensure that cleaning validation practices are followed properly.
Learn More About Cleaning Validation at CfPIE
Do you or your facility staff need certified training on cleaning validation and other related topics? Get in touch with CfPIE. Our course: “Best Practices for an Effective Cleaning Validation Program,” provides practical guidance on regulatory compliance.
Whether you need drug or medical device cleaning validation, you will find immense value in this course taught by
certified instructors in the medical industry.
Register today to get started!
Blog Categories
Stay Informed


